Clinical trial pain-point
Labelling de-identified studies is a tedious and manual process in most clinical trials, and with all other manual processes it is prone to human error. Mislabeling of studies can have detrimental effects on overall study outcomes.
SliceVault solution
SliceVault uses artificial intelligences (AI) to ensure CT studies are labelled correctly.

Study A
Study B

Same subject
Not same subject
Automated visual inspection
When correct labelling cannot be determined based on DICOM tags, because studies have been de-identified in most clinical trials, we resort to visual inspections. Sometimes this is an easy task to detect that two CTs are obtained from different subjects, for example when they differ in size, gender or if only one subject has a hip prosthesis. In other situations, incorrect labelling can be easily overlooked, especially since most studies are labelled correctly and the image reader is focused primarily on endpoint assessment
SliceVault comes with an AI-based visual inspection tool where incorrect labelled CT studies are flagged automatically.
Step 1: Organs and skeletal structures are segmented automatically in CT studies by SliceVault AI.
Step 2: A surface mesh is extracted for each structure using the Surface Nets technique and meshes are aligned using the Iterative Closest Point method.
Determining anatomical match
With the aligned meshes, the anatomical match index describing the similarity between studies is calculated to determine whether different CT studies belong to the same subject or not.
Example alignment of meshes of left scapula

Same subject
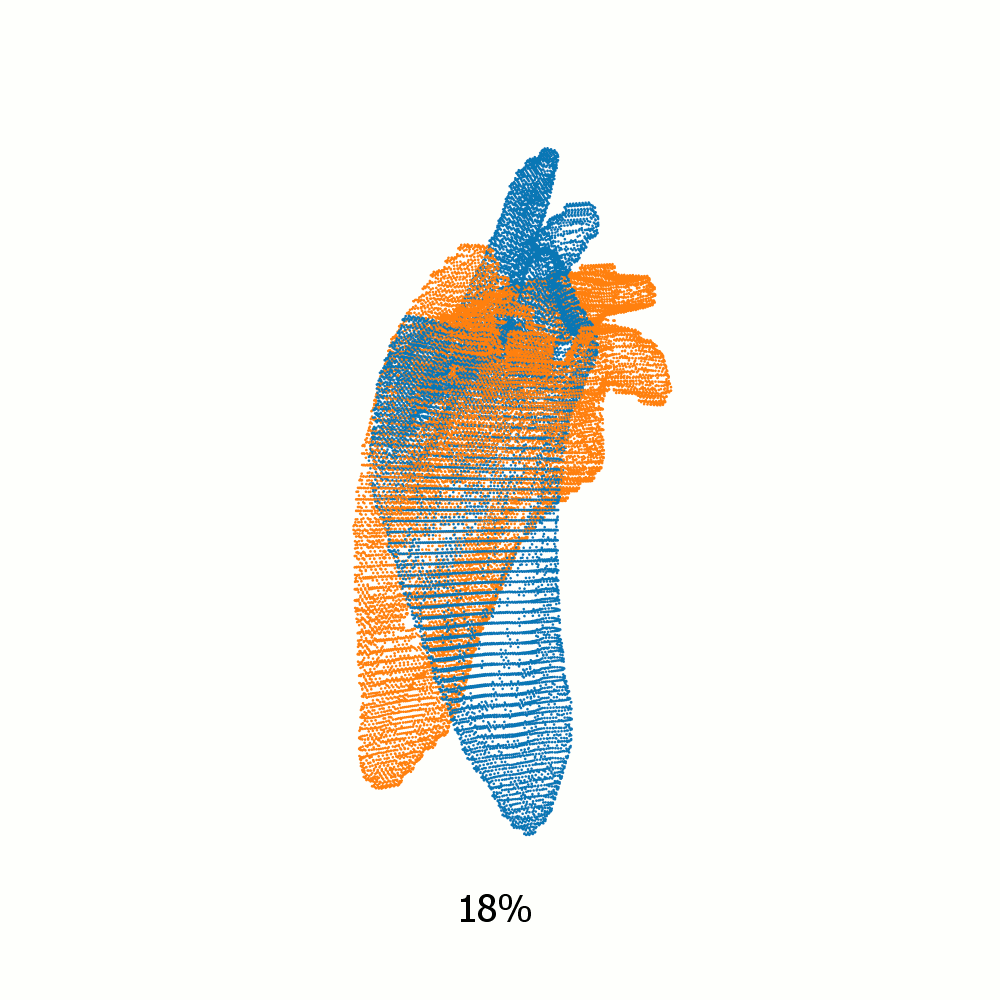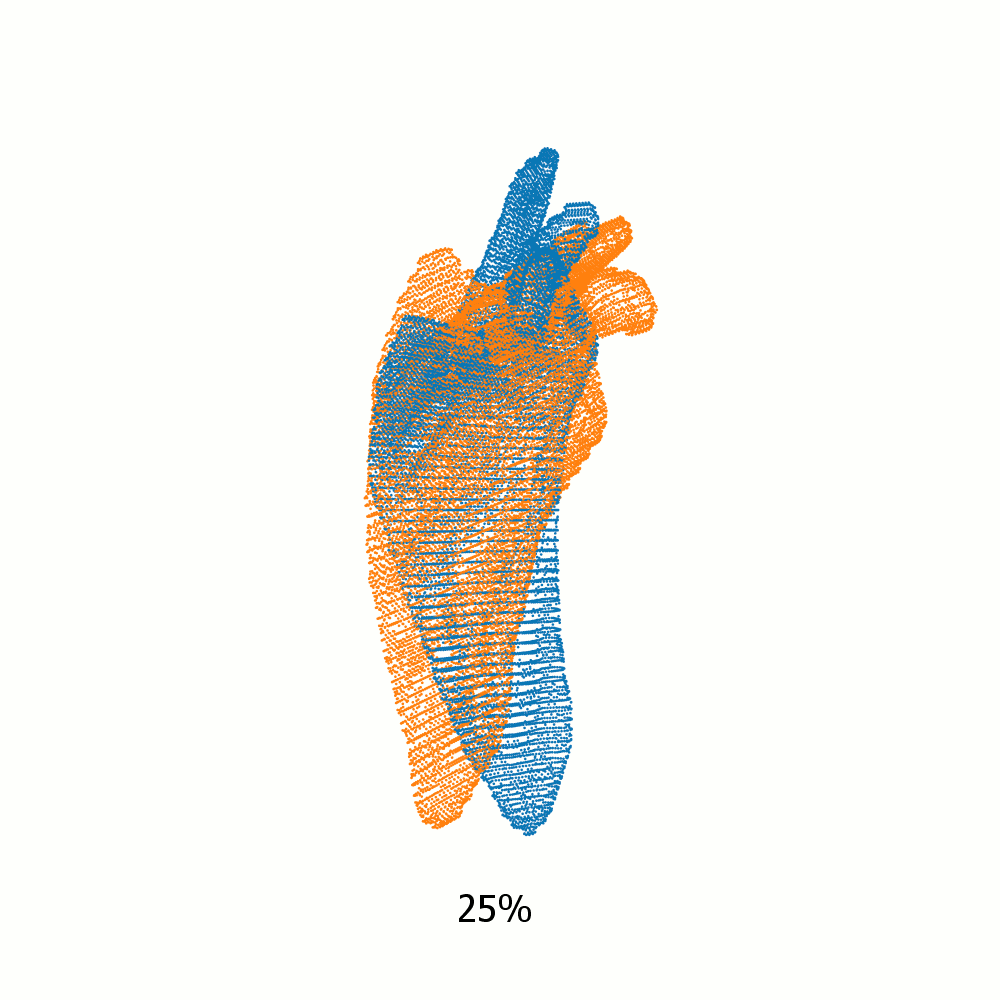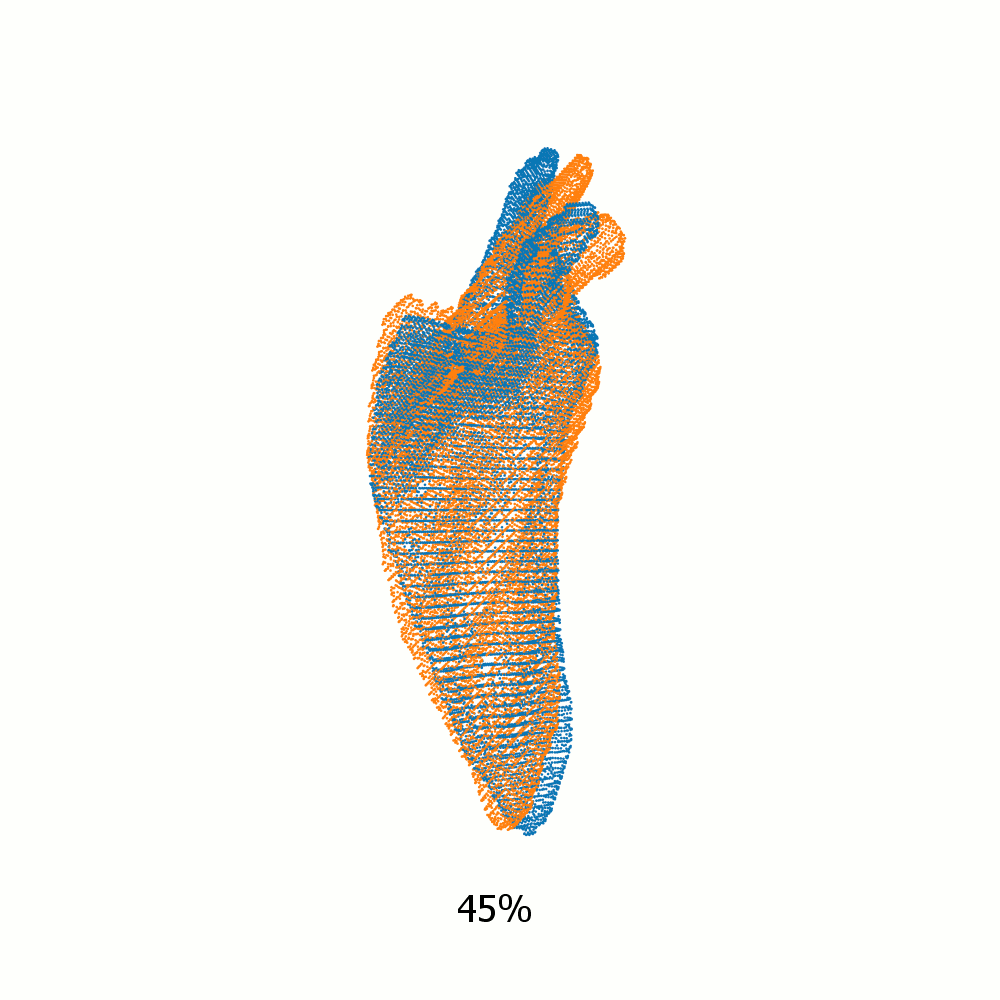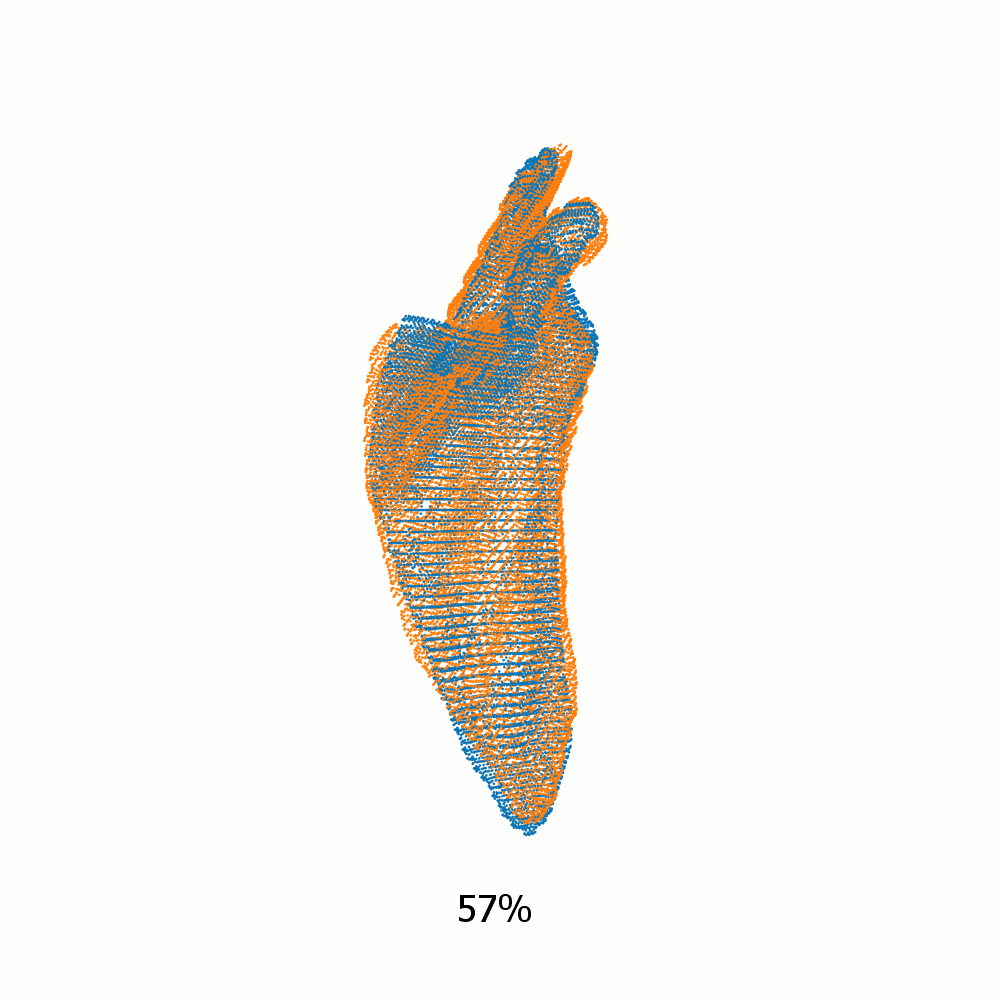
Not same subject