RECIST 1.1 Reporting Software for Oncology Trials
3 ways SliceVault’s RECIST 1.1 module delivers superior results and streamlined efficiency
01
Designed for clinical research workflows
SliceVault streamlines the complex processes of lesion measurement, tracking, and response categorization, ensuring precise and consistent assessments across all trial visits. With SliceVault, navigating the complexities of RECIST 1.1 reporting is simplified, enabling more efficient, timely, and precise outcomes.



02
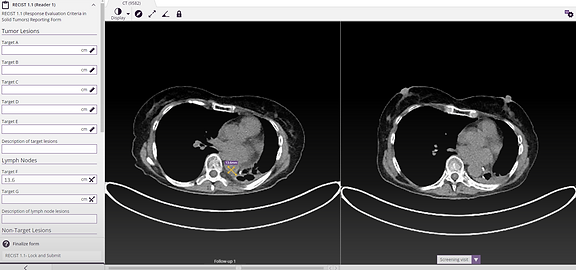
Effortless RECIST 1.1 reporting
SliceVault's RECIST 1.1 module makes it easy to annotate lesions and transfer measurements directly into the reporting forms, significantly reducing manual data entry and increasing the reliability of your data.
03
From images to reports in one click
SliceVault automatically extracts data directly from annotated images into customizable report templates, ready for electronic signature and distribution in compliance with FDA 21 CFR Part 11.
This streamlined approach not only simplifies the reporting process but also ensures your reports adhere to the strictest regulatory compliance requirements, making them robust and reliable for clinical trial documentation.

4 reasons why clinical teams choose SliceVault
01
Built for clinical trial workflows

02
Integrated review and reporting

03
Scales with your study

04
Compliance you can trust

8 powerful features clinical teams rely on
From upload to audit, SliceVault streamlines every step of imaging in clinical trials. These core features make your workflow faster, safer, and fully compliant.
Simple submission
Drag-and-drop image upload with structured visit selection to ensure correct mapping in trials.
Automatic de-identification
DICOM tags are automatically scrubbed or retained per protocol, protecting patient privacy while maintaining essential study data.

Automatic quality checks
System-level checks flag missing or corrupted files before they enter the workflow.

Manual quality control
Dedicated QC user role allows teams to verify image parameters, ensure compliance, and document findings directly in the platform.

Integrated DICOM viewer
A built-in cloud viewer supports structured reads with combined reading and reporting capabilities.

Audit logging & compliance
Every action is recorded in a secure audit trail for full regulatory compliance and inspection readiness.

Role-based access control
Granular permissions ensure only authorized users can upload, QC, read, or manage data.

Secure, scalable cloud
Built on Microsoft Azure with redundancy, encryption, and disaster recovery, ensuring data is safe and globally available.
What our clients say
Megan Henning
Industry Operations Manager | Fivos, Inc.
SliceVault has significantly streamlined the image upload process and core lab analysis for our projects
SliceVault has significantly streamlined the image upload process and core lab analysis for our projects. The software's user-friendliness has greatly reduced manual effort, particularly in ensuring image protocol compliance and automatic removal of PHI.
The ability for the core lab to analyze, measure, and input data directly within the software has been particularly beneficial, offering full transparency for our projects. Additionally, the DTF capabilities for sites to input pertinent project data have centralized both data capture and image uploads, which has streamlined the process resulting in less time for image upload.
The support we've received from SliceVault over the past few years has been exceptional. Questions and concerns are consistently addressed in a timely manner, and the SliceVault support team is always proactive in suggesting and implementing improvements to the software or our projects to enhance efficiency. We truly enjoy working with the SliceVault team!
Albert Yen
Founder & CEO | tWAN Biotech
It’s always a pleasure to work with them
The SliceVault team is highly responsive, professional and reliable addressing user support requests. The platform simplifies our trial monitoring with its comprehensive, downloadable reports. It’s always a pleasure to work with them.
Senior Clinical Research Project Manager Anova Evidence
SliceVault is easy to navigate and at a price point that many clients deem attractive
Before SliceVault we did not have an imaging repository in place and other repositories were either difficult to manage or extremely expensive. SliceVault is easier to navigate and at a price point that many clients deem attractive.
The color wheel feature has helped us clearly view perfusion scan images to identify the areas that have active tumor activity. This feature is paramount when ruling out progression versus pseudo progression. It makes the difference in whether a study participant can continue on trial or not.
The support is speedy, reliable and professional
Your story could be here!
Jim Bakker
Clinical Associate | Amber Implants
The support of the SliceVault team is truly amazing
The system is very straightforward when it comes to collecting data and analyzing the metadata of all the uploaded images. Also, the functionality to easily adjust of add Dicom-tags helps us greatly with forwarding our images to the participating core lab for measurement in a orderly fashion.
The most outstanding function for our clinical trial is the simple but effective overview of all the visits of our study subject in one clear interface. Furthermore, the colour coding of the visits is very useful when it comes to keeping an overview of what has to be done with every visit.
The support of the SliceVault team is truly amazing. Responsiveness is often less than a day and due to the nature of the platform, problems can be solved straight away in an interactive way.
Joe El-Aklouk
Director - Product Management | Ferronova
The automated de-identification is a big help which simplifies compliance
Using SliceVault to de-identify and store MRI, CT, and PET/CT scans has made things much smoother. Having the scans uploaded into one secure, organised system means less manual effort to track files. Reducing errors (tags, metadata, etc.) has saved time and hassle.
The automated de-identification is a big help which simplifies compliance. The high-risk lymph node tagging on MRI scans is also especially useful in our trial. Exporting data is easy when we need to share or analyse it outside the system.
Excellent. When we’ve encountered issues or had questions, the support has been responsive, clear, and helpful. It makes a difference knowing the team is there when needed.


Prefer to talk to us directly?
We are available by phone and email if you would like to learn more, have any questions, or would like a tour of the system. Leave your name and company email in the form below, call us at +46 73 654 9554, or email us at contact@slicevault.com and we will get back to you promptly.


