Why clinical teams choose SliceVault
Designed for clinical research workflows
SliceVault is purpose-built for imaging in clinical trials. From upload to compliant reporting, every step follows the way research teams actually work.
Sites can automatically de-identify and securely transfer images, QC reviewers can validate them, and readers can report results using structured templates. All in one seamless cloud workflow.




Compliance and security,
built in
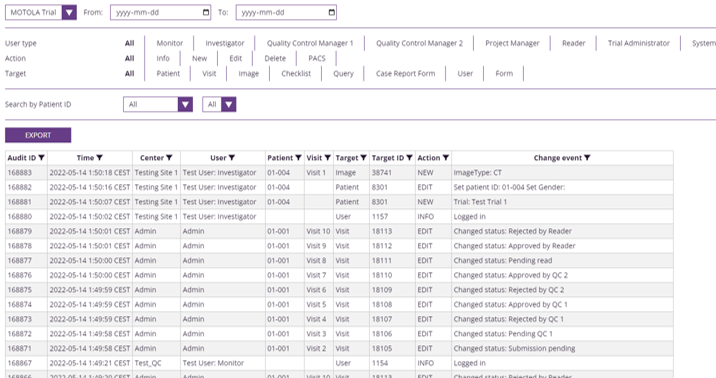
With SliceVault, compliance isn’t an afterthought - it’s part of the foundation. Every upload, edit, and review is automatically logged in a 21 CFR Part 11 ready audit trail.
Data is encrypted in transit and at rest, with role-based permissions that keep global collaborations secure and inspection-ready.


Trusted results and efficiency
Clinical teams worldwide trust SliceVault to cut down manual work and reduce errors. Reports can be generated in one click, exports are ready for oversight, and image turnaround times are faster.
By streamlining imaging workflows, SliceVault helps research projects stay on schedule and in compliance.

Trusted by leading organizations worldwide
Streamlined workflows in one platform
SliceVault simplifies submissions with automatic de-identification, ensures quality with manual and automated QC checks, and delivers central reading workflows tailored to your project in one secure cloud platform.
Submission
QC review
Central Reading
What our clients say



















